|
|
|
|
|
|
| |
|
|
| |
Lithium-ion batteries made us mobile, rechargeable, and safe(r). |
|
| |
|
|
|
| |
|
|
| |
|
|
| |
| |
|
|
| |
(Illustration by Brad Yeo) |
|
| |
|
|
|
|
| |
|
|
|
| |
|
|
| |
Congratulations to John B. Goodenough, SM'50, PhD'52, who shares the 2019 Nobel Prize in Chemistry for his pioneering work developing lithium-ion batteries. His research helps lay the foundation for a wireless, fossil-fuel-free society. |
|
| |
|
|
|
| |
|
|
| |
Batteries, first invented in 1800, work by converting chemical energy into electricity. They have two electrodes made of different metals--the negative anode and positive cathode--separated by an electrolyte. When the electrolyte reacts with the anode metal, it creates ions, which migrate to the cathode inside the battery, and electrons, which travel through the wire outside the battery, powering your smartphone or cordless drill. The electrolyte is reacting with the cathode, too, making it eager to suck up the ions and electrons. |
|
| |
|
|
|
| |
|
|
| |
The first rechargeable batteries, which use external electricity to reverse the reactions, were lead-acid. They're best known as car batteries: cheap and dependable but heavy and low voltage. Early cars ran entirely on these batteries, but eventually they were used only to start gas engines. |
|
| |
|
|
|
| |
|
|
| |
By the 1960s, oil shortages and smog recharged interest in all-battery-powered vehicles. Ford invited physicist John Goodenough, SM'50, PhD'52, to monitor development of a sodium-sulfur car battery. It was deemed impractical, but the experience sparked Goodenough's interest. |
|
| |
|
|
|
| |
|
|
| |
|
|
| |
| |
|
|
| |
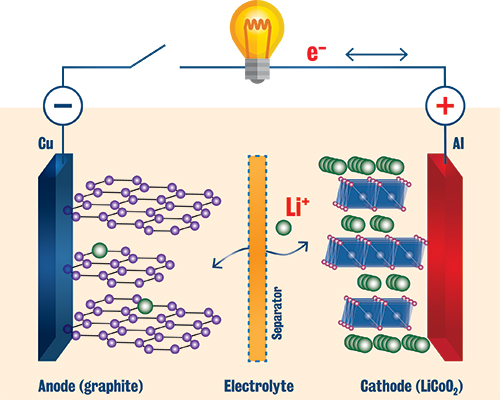
Schematic of a lithium-ion battery. (Adapted with permission from John B. Goodenough and Kyu-Sung Park, Journal of the American Chemical Society, 2013, 135 (4), pp 1167–1176. DOI: 10.1021/ja3091438. Copyright © 2013 American Chemical Society.) |
|
| |
|
|
|
|
| |
|
|
|
| |
|
|
| |
Exxon patented the first lithium battery, created by Stanley Whittingham in 1976. The battery had low weight and large voltage capacity, but there was a drawback: as the lithium anode disintegrated, it tended to explode. |
|
| |
|
|
|
| |
|
|
| |
Goodenough, by then at Oxford, improved the design with a more stable, layered cathode that holds lithium ions in its framework. Chemist Akira Yoshino took Goodenough's layered cathode, paired it with his own layered anode, which also holds lithium ions, and created the first lithium-ion battery, commercialized in 1991. |
|
| |
|
|
|
| |
|
|
| |
In October Whittingham, Goodenough, and Yoshino were awarded the 2019 Nobel Prize in Chemistry for revolutionizing mobile technology. |
|
| |
|
|
|
|
|
|
|
| |
|
|
| |
|
|
|
| John Goodenough is now exploring more sustainable options, like replacing lithium with nearly limitless sodium. |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
| |
|
|
|
| National labs use X-rays to peer inside lithium-ion batteries while in use to better understand how they work. |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
| |
|
|
| |
| |
|
|
| |
John B. Goodenough discusses battery innovation. |
|
| |
|
|
|
|
| |
|
|
|
| |
|
|
| |
Watch 2019 Nobel laureate John B. Goodenough, SM'50, PhD'52, discuss his late-blooming science career, family life, and hope for a sustainable future in this video from 2016. And keep an eye out for the late Millie Dresselhaus, PhD'59, in her signature red jacket. |
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
| |
Elemental:
It's the periodic table's 150th anniversary, and it's getting heavy.
|
| |
|
|
|
|
| |
Win this mug:
Forward µChicago and we'll enter you in our monthly drawing.
|
| |
|
|
|
|
|
|
|
|
|
|
|
|